Scientific papers
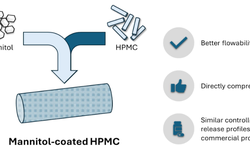
Background: Hypromellose (HPMC) has been utilized previously to regulate drug release in mini-tablets. However, due to suboptimal flow characteristics, the production of mini-tablets with elevated HPMC levels presents a challenge. The Dow Chemical Company has introduced directly compressible (DC) HPMC grades.
Objective: To compare the characteristics of HPMC DC (METHOCEL™ K4M and K100M) with regular (REG) HPMC grades.
Methodology: Assessment of particle size distribution and flowability of HPMC REG and DC. Generation of 3 mm mini-tablets, incorporating hydrocortisone or theophylline as model drugs and 40% w/w HPMC DC or REG. Production of mini-tablets containing HPMC DC grades using a rotary press simulator at forces between 2–4 kN and speeds of 5, 10, 15, or 20 rpm, while HPMC REG mini-tablets were manually produced.
Results and Discussion: The enhanced flowability of HPMC DC grades, characterized by a narrower particle size distribution and larger particle sizes, facilitated the simulated large-scale production of mini-tablets with satisfactory weight uniformity (CV 1.79–4.65%). Automatic manufacturing of mini-tablets containing HPMC REG was impractical due to the poor flowability of the formulations. Drug release from mini-tablets comprising HPMC DC and REG exhibited comparability. Mini-tablets containing HPMC DC demonstrated higher tensile strength compared to those made with HPMC REG. Mini-tablets produced with HPMC DC at different compression speeds displayed similar drug release profiles.
Conclusions: Successful production of extended-release mini-tablets was achieved with the use of HPMC DC. The drug release rate was not affected by the different HPMC DC grades (K4M or K100M) or production speed.
Comments
No comments posted yet.
Add a comment