Scientific papers
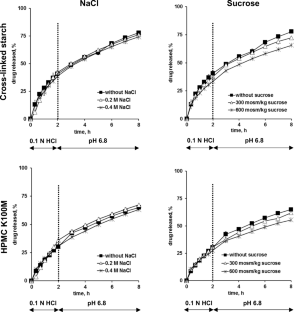
The objective of this research was to assess the viability of utilizing cross-linked pregelatinized potato starch (marketed as PREGEFLO ® PI10) as a matrix former for controlled release tablets. Various types of tablets, containing diprophylline, diltiazem HCl, or theophylline, were produced through the direct compression of binary drug/polymer blends. The drug content ranged from 20 to 50%. As an alternative, two grades of hydroxypropyl methylcellulose (HPMC K100LV and K100M) were examined as potential matrix formers. Drug release was gauged across different release media using diverse experimental setups, encompassing 0.1 N HCl, phosphate buffer pH 6.8, and water. Additionally, the media included varying amounts of NaCl, sucrose, ethanol, or pancreatin, along with simulations of fasted state gastric fluid, fed state gastric fluid, fasted state intestinal fluid, fed state intestinal fluid, and conditions simulating the colon in both healthy subjects and those with Crohn’s disease. The USP apparatuses I/II/III were employed under various operating conditions, potentially coupled with additional mechanical stress simulation. Notably, the drug release kinetics showed minimal alteration under the investigated environmental conditions for tablets based on cross-linked pregelatinized potato starch, similar to HPMC tablets. However, unlike the latter, the starch-based tablets retained their shape to a considerable extent upon exposure to the release media, primarily increasing in size during the observation period. Additionally, water penetration into the systems was noticeably less pronounced. Therefore, the explored cross-linked pregelatinized potato starch exhibits intriguing potential as a matrix former in controlled release tablets.
Comments
No comments posted yet.
Add a comment