Scientific papers
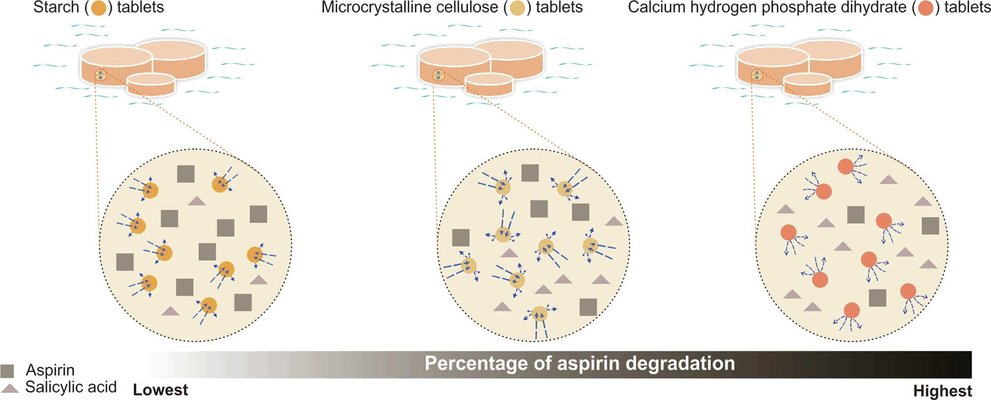
The interaction between excipients and moisture can significantly impact product stability. This study aimed to investigate the influence of incorporating excipients with varying moisture interactions into formulations containing a moisture-sensitive drug, aspirin. Aspirin formulations were developed using maize starch (MS), microcrystalline cellulose (MCC), and calcium hydrogen phosphate dihydrate (DCP). The excipients were assessed for their intrinsic moisture content and water activity. Tablets, produced at different compression pressures, were subjected to 40 °C and 75% relative humidity for a specific duration, and subsequently analyzed for aspirin degradation. The results indicated that, despite having higher moisture content, MS tablets exhibited lower aspirin degradation compared to MCC and DCP tablets, attributed to MS's relatively low water activity. Conversely, DCP, with high water activity, showed greater aspirin degradation despite its low moisture content. The impact of tablet porosity on aspirin degradation was minimal, emphasizing the limited influence of physical spatial constraints on moisture distribution. These findings suggest that excipients with high water-retentive capacity could potentially serve as internal tablet desiccants, acting as moisture scavengers. The study underscores the significance of considering water activity in preformulation studies when selecting excipients.
Comments
No comments posted yet.
Add a comment