Scientific papers
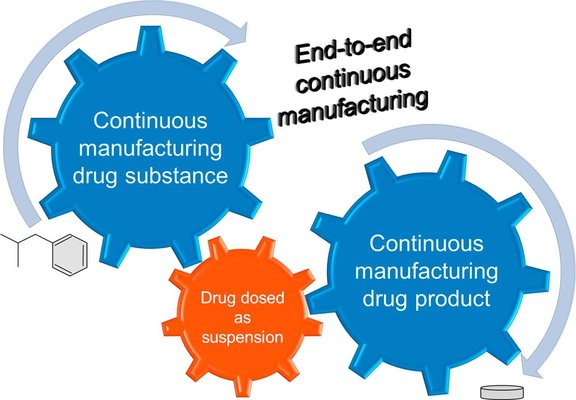
This study aimed to explore the integration of continuous manufacturing for drug substance and drug product in an end-to-end continuous manufacturing process. The key step in this integrated approach involved dosing the active pharmaceutical ingredient (API) as a suspension into a twin-screw wet granulation process. To achieve this objective, a model drug substance (ibuprofen) was introduced as a concentrated aqueous suspension (50% w/w) into a twin-screw granulator and compared with the traditional method of solid feeding to achieve a target ibuprofen load of 60% w/w in the formulation. The granulation and compaction behaviors were analyzed to assess the impact of feeding the API as a suspension in twin-screw wet granulation on the critical quality attributes of the drug product. The study demonstrated that the ibuprofen suspension feed yielded comparable results to the ibuprofen dry blend feed in twin-screw wet granulation. In addition to facilitating end-to-end continuous manufacturing, utilizing API suspension feed in twin-screw wet granulation could provide several additional benefits, including enhanced manufacturing efficiency by eliminating the drying step for the API or overcoming processing challenges associated with the bulk properties of the API powder, such as poor flowability.
Comments
No comments posted yet.
Add a comment