Scientific papers
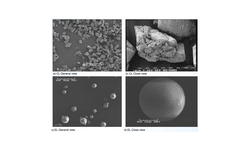
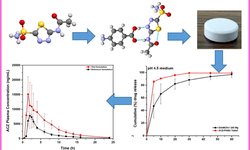
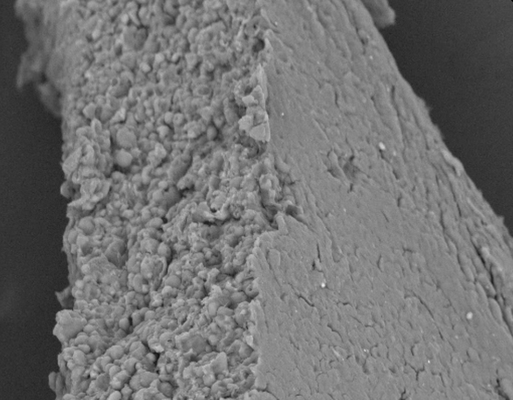
This study tackles the challenge of creating an affordable, patient-friendly alternative to antibiotics for gastrointestinal applications, utilizing bacteriophages. The research investigates the possibility of producing an enteric solid dosage form incorporating a salmonella-specific Myoviridae phage, Felix O1, enclosed in spray-dried trehalose/Eudragit microparticles. The spray-dried powder is further processed by combining it with magnesium stearate to facilitate tablet manufacturing through direct compression. The paper offers a comprehensive assessment of the tablets, including measurements of phage viability during tablet fabrication with various compression settings, and evaluations post-tablet disintegration, dissolution, and friability.
Phage viability measurements encompass storage stability testing of spray-dried powders and tablets in sealed vials at 4 °C, 20 °C, and 30 °C under different humidity conditions (0%, 50%, and 65% RH). The recommended compression force range for a standard 10 mm diameter tablet was determined to be 10–15 kN. The most favorable conditions for tablet storage, resulting in a ~1 log loss in titre over a six-month period, were found to be at 4 °C/0% RH. Storage at higher temperatures and exposure to high humidity levels led to a notable decline in phage viability. The paper sheds light on the challenges in developing phage formulations suitable for direct-compression tableting, aiming to provide phages protection against temperature and humidity conditions that do not necessitate a cold supply chain.
Comments
No comments posted yet.
Add a comment