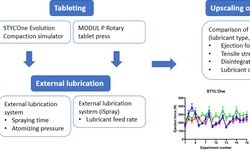
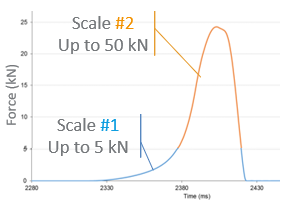
Scientific papers
In the development of pharmaceutical dosage forms, the choice of excipients plays a pivotal role in both preformulation and formulation research. The physical, mechanical, and chemical attributes of excipients exert a significant influence on the final product, as well as on various formulation parameters such as disintegration, dissolution, and shelf life. Consequently, numerous studies have been conducted to assess the impact of drug-excipient interactions on the overall formulation.
This article provides a comprehensive review of information regarding the physical and chemical instability of excipients and their compatibility with the active pharmaceutical component in solid oral dosage forms throughout various drug manufacturing processes. The detailed discussion delves into the repercussions of these interactions on the drug formulation process. Additionally, various examples of excipients employed in solid oral dosage forms are presented to elucidate different drug-excipient interactions.
Comments
No comments posted yet.
Add a comment