Scientific papers
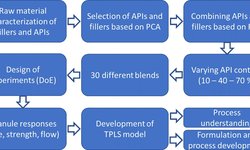
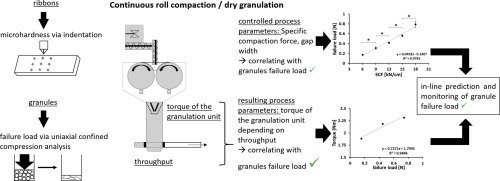
A crucial quality aspect of solid oral dosage forms lies in their firmness and capacity to endure breaking or grinding. When aiming for continuous manufacturing, it becomes valuable to assess the material hardness at various production stages. By manipulating controlled process parameters such as specific compaction force, roll speed, or gap width in roll compaction/dry granulation, it becomes possible to anticipate the hardness of resulting ribbons and granules. In this study, a novel approach involves utilizing two interdependent process parameters—torque of the granulation unit and throughput of material—to predict granule failure load. The study also observed the in-line escalation of granule hardness as the specific compaction force increased during the compaction process. This breakthrough paves the way for real-time monitoring of material hardness, enabling its incorporation into feedback and feedforward control loops for prospective continuous manufacturing processes.
Comments
No comments posted yet.
Add a comment