Scientific papers
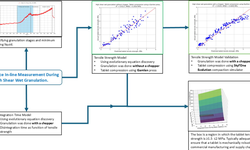
The objective of this investigation was to explore powder mechanics during compression by analyzing data derived from force-displacement (F-D) curves. The study employed the Kawakita model of powder compression analysis to compare the pressure-volume reduction relationship of drug powders with the F-D curves. Six model drugs (metronidazole, metformin, secnidazole, ciprofloxacin, norfloxacin, and mebeverine) were subjected to experiments, undergoing compression at various pressures in both non-processed and processed (using a roller compactor) forms. The results reveal a resemblance between the F-D curves and a modified version of the Kawakita model. This resemblance facilitates the calculation of two crucial powder parameters, namely "a" (maximum powder volume reduction) and "Pk" (pressure required to achieve half of the maximum volume reduction) directly from the F-D curves. Importantly, unlike the conventional Kawakita model, there is no need to compress powders into tablets at different compression forces.
Comments
No comments posted yet.
Add a comment