High-dose modified-release formulation of a poorly soluble drug via twin-screw melt coating and granulation
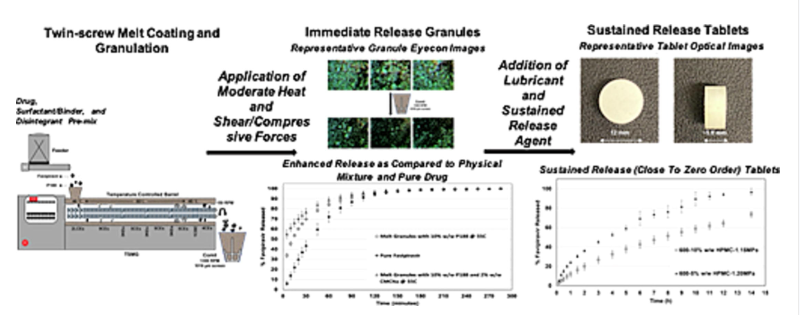
Favipiravir, an antiviral drug for COVID-19 with poor water solubility, is formulated using a low-cost melt coating and granulation method. High-dose tablets (600 mg and 800 mg) with desired release profiles are developed, minimizing excipient burden. The process involves twin-screw melt coating and granulation (MCG) using Poloxamer P188 as a binder and surfactant, creating granules with high solubility and tabletability. These granules are then blended with additional ingredients and compacted into controlled-release tablets. This method is effective for manufacturing high-dose modified release formulations for low solubility drugs used in treating various conditions.
Comments
No comments posted yet.
















