The effect of formulation variables on the manufacturability of clopidogrel tablets via fluidized hot-melt granulation
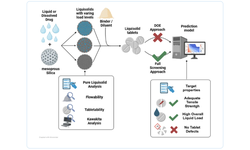
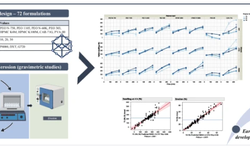
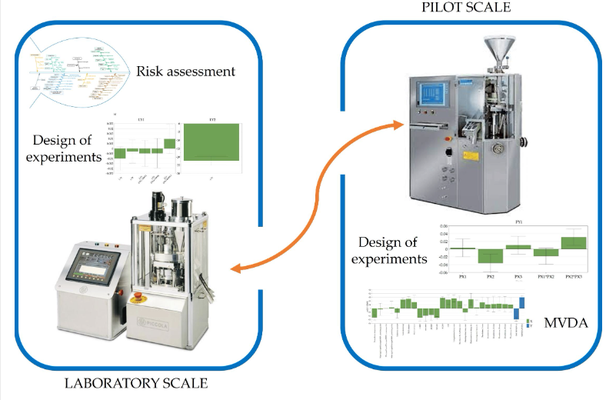
This study investigates the challenges faced by solid pharmaceutical formulations containing class II active pharmaceutical ingredients (APIs) due to limited solubility, which affects their behavior in the body. Computational tools and data mining techniques offer insights into product performance, complementing traditional methods and assisting in scale-up decisions. Through the use of design of experiments (DoE) and exploratory data analysis (MVDA), the study examines fluidized hot-melt granulation manufacturing technology. Findings reveal significant impacts of granulation temperature, time, and Macrogol type on product quality attributes, such as resistance to crushing and dissolution profiles. The equipment and materials used for the study are also detailed, encompassing both laboratory and pilot-scale experiments.
Comments
No comments posted yet.