Scientific papers
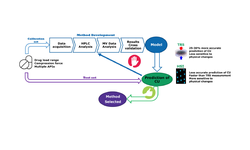
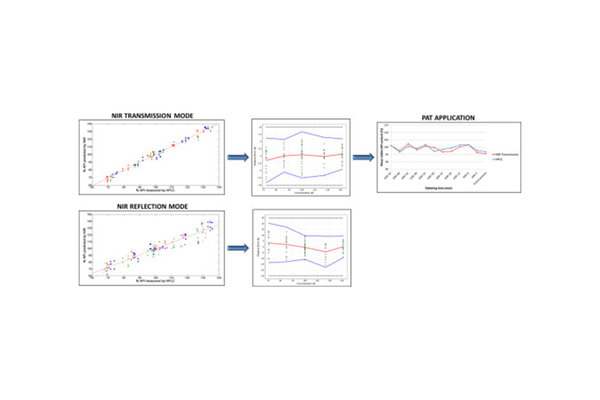
This study aimed to establish Near-Infrared (NIR) methods for determining the active content in non-coated pharmaceutical tablets produced from a proportional tablet formulation. These NIR methods were developed to monitor the active content of tablets throughout the tableting process. Initially, methods were created in both transmission and reflection modes to quantify the API content of the lowest dosage strength. Subsequently, these methods underwent a comprehensive validation covering a concentration range of 70–130% of the target active content, employing the accuracy profile approach based on β-expectation tolerance intervals. The model using the transmission mode demonstrated superior predictive accuracy for the correct active content compared to the reflection mode. Nevertheless, the reflection mode's ability to quantify the API content in the highest dosage strength was also evaluated. Additionally, the NIR method based on the transmission mode was effectively employed for at-line monitoring of tablet active content during the tableting process, enhancing understanding of API content throughout the process. This enhanced quality control aligns well with the Quality by Design (QbD) concept. Finally, the successful transition of the transmission model from off-line to an on-line spectrometer was explored.
Comments
No comments posted yet.
Add a comment