Scientific papers
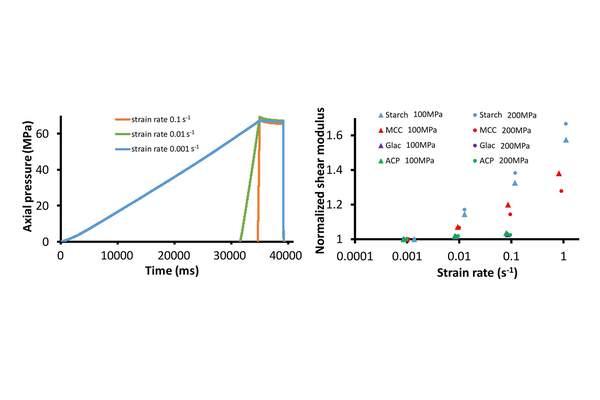
The variation in compaction properties of powders with compaction speed, known as strain rate sensitivity (SRS), is a common occurrence in the pharmaceutical tablet manufacturing process. However, various phenomena, such as friction, viscoelasticity, viscoplasticity, or air entrapment, can be accountable for SRS.
In this study, an innovative experimental methodology was developed to specifically characterize the viscoelasticity of tablets using a compaction simulator. After multiple compressions, tablets were elastically loaded at different constant strain rates. This approach allowed the measurement of the apparent bulk and shear moduli as a function of the strain rate. The methodology was successfully applied to microcrystalline cellulose (MCC), Starch, Lactose monohydrate (GLac), and Anhydrous Calcium Phosphate (ACP). As anticipated, no significant evolution of the moduli was observed for Lac and ACP. In contrast, for MCC and Starch, both shear and bulk moduli were found to increase with the strain rate. The viscoelastic behavior was effectively modeled using Prony series, and the model parameters were assessed through inverse identification using an analytical model and finite element analysis.
Comments
No comments posted yet.
Add a comment