Scientific papers
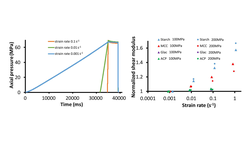
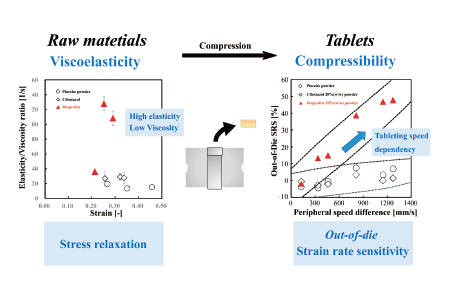
This study investigated a quantitative evaluation method to assess the impact of tableting speed on the compression properties of pharmaceutical powders. Cilostazol and ibuprofen served as active pharmaceutical ingredients (APIs), blended with lactose monohydrate and microcrystalline cellulose. The raw materials were evaluated for viscoelasticity, and stress relaxation tests were conducted to determine the apparent viscosity and elasticity coefficients of the placebo and the two APIs. Tablets were then prepared using both a compaction simulator and a rotary tablet press, covering a range of tableting speeds from laboratory to commercial scales. The in-die and out-of-die strain rate sensitivity (SRS) indices were determined to gauge compressibility and compactibility. The results indicated that the out-of-die SRS exhibited higher sensitivity compared to the in-die SRS. For ibuprofen 20% powder, characterized by high elasticity and low viscosity, the out-of-die SRS ranged from 13.3% to 47.9%, while the placebo and cilostazol 20% (w/w) powder showed values below 7.5%. A peripheral speed difference exceeding 300 mm/s during the out-of-die SRS proved sensitive in detecting capping tendencies. Cilostazol, with lower elasticity and higher viscosity than ibuprofen, was tested across API concentrations of 5–40%, resulting in compressibility SRS values below 5% for all API concentrations. In contrast, the compressibility SRS of ibuprofen ranged from 4.8% to 81%, depending on the API concentration. Utilizing the compressibility SRS as an index allowed for extracting the tableting speed-dependent compressibility characteristics of the API from the powder mixtures containing the API.
Comments
No comments posted yet.
Add a comment