Scientific papers
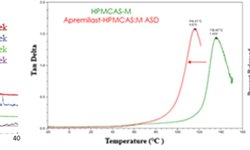
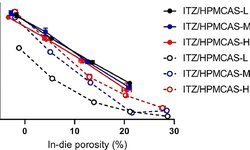
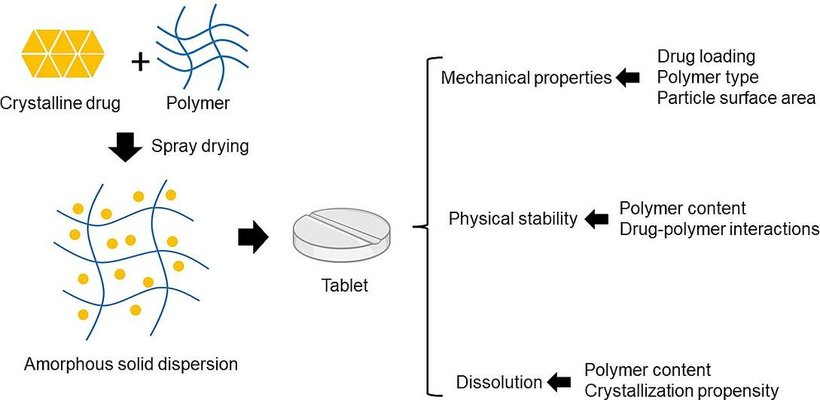
The amorphous solid dispersion (ASD) method is a well-established approach to enhance the solubility and bioavailability of poorly soluble drugs. Although a significant number of ASD products are in tablet form, the impact of common polymers and drug loading on the manufacturability of ASD tablets has been insufficiently explored. This study specifically focuses on examining spray-dried ASDs from a tableting standpoint, assessing their physiochemical and mechanical properties. Itraconazole (ITZ) and indomethacin (IND), with drug loadings ranging from 10% to 50%, were prepared using two representative polymers: hydroxypropyl methylcellulose acetate succinate (HPMCAS) and polyvinylpyrrolidone (PVP). Our findings revealed that increasing drug loading led to a decreased surface area in ITZ-HPMCAS, IND-HPMCAS, and IND-PVP ASDs. However, this trend was not observed in ITZ-PVP dispersions, possibly due to morphological differences. Compaction results demonstrated that tabletability improved with decreasing drug loadings, except for ITZ-PVP dispersions. A partial least square analysis highlighted particle surface area as the key factor influencing the tensile strength of ASD tablets. Furthermore, our study disclosed that ITZ-PVP ASDs exhibited the poorest release profiles and stability performance. The comprehensive exploration from characterizing ASD particles to analyzing their compaction behavior and investigating drug release and physical stability provided profound insights into the critical attributes for downstream processing of amorphous pharmaceuticals.
Comments
No comments posted yet.
Add a comment