Scientific papers
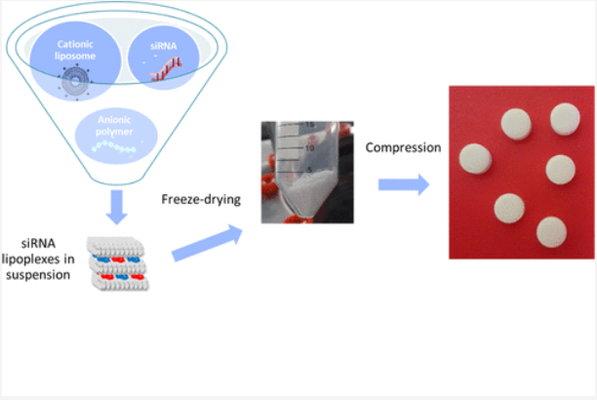
Presently, the majority of nonviral nucleic acid vectors are formulated as colloidal suspensions primarily administered parenterally. This formulation and administration method impose significant constraints, such as vector size limitations and the need for sterile preparations. The tablet form offers the advantage of easy oral administration, well-received by patients. For nucleic acid vectors, a dry form represents a notable improvement in stability. We investigated the tabletability of a liquid suspension of nucleic acid vectors using an optimized lipid-based small interfering RNA (siRNA) delivery system. Freeze-drying conditions were optimized to preserve both the gene-silencing efficacy of the formulated siRNAs and the supramolecular structure of the lipid particulate system. Gene-silencing efficacy was assessed on luciferase-expressing cells, and the structure of the siRNA vector in freeze-dried and tablet forms was analyzed using small-angle X-ray scattering (SAXS) synchrotron radiation. The freeze-dried powders were mixed with excipients essential for smooth compression, ensuring a consistent matrix supply and reducing friction. Compression was performed using a rotary press simulator, enabling comprehensive monitoring of compression conditions. Post-compression, the formulated siRNAs retained over 60% of their gene-silencing efficacy. Specific SAXS signals within the tablets indicated the preservation of lamellar and cubic phases from the initial liquid suspension, and these phases were restored upon resuspension of siRNA vectors through tablet disintegration. These findings demonstrate the preservation of bilayer lipid structures despite the mechanical stresses imposed by compression. While such preservation was anticipated after freeze-drying, to our knowledge, this study is the first to show that siRNA delivery systems can maintain their efficacy and structure after mechanical stress, such as compression. This discovery holds promise for the oral administration of siRNA as an alternative to parenteral administration.
Comments
No comments posted yet.
Add a comment