Scientific papers
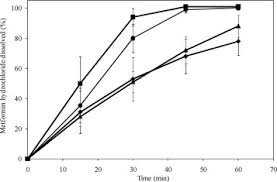
The aqueous solubility of metformin (pKa: 2.8 and 11.5) within the pH range of 1.2–6.8 is 300 mg/mL, suggesting that the dissolution of metformin hydrochloride tablets should exhibit pH independence. However, 850 mg metformin hydrochloride tablets displayed a slower dissolution rate in pH 1.2 and 4.5 media compared to pH 6.8 medium. It is postulated that the additional protonation of metformin at acidic pH levels results in increased solvation and a larger hydrodynamic radius, leading to slower diffusion and dissolution. This hypothesis finds support in the observation that cationic metformin and anionic sodium lauryl sulfate (SLS) at 0.1% (w/v) formed an insoluble salt (1:2 molar ratio) at pH 1.2 and 4.5 but not at pH 6.8. The presence of SLS at 0.01% (w/v) in all three media enhanced metformin dissolution. Slower metformin dissolution in pH 1.2 and 4.5 media with SLS can be attributed to the formation of metformin–lauryl sulfate (Met–LS) (1:2 and 1:1) ion pairs, which are more hydrophobic than Met–LS (1:1) ion pairs at pH 6.8. Diffusion-ordered spectroscopy nuclear magnetic resonance revealed slower metformin diffusivity in pH 4.5 with 0.05% (w/v) SLS. Despite the lower diffusivity of metformin-LS ion pairs, the improved metformin wetting by SLS outweighed this factor, as similar enhancement in dissolution was noted with 0.5% (w/v) nonionic polysorbate 80.
Comments
No comments posted yet.
Add a comment














