Scientific papers
A study was undertaken to gain a mechanistic understanding of the dissolution of metformin hydrochloride, a highly soluble cationic drug, in the presence of anionic surfactants known as sodium alkyl sulfates. These surfactants exhibited no impact on the drug's saturated solubility but effectively reduced the surface tension of the dissolution media, with the extent of reduction correlating with the alkyl chain length. The influence of these surfactants on tablet wetting, as assessed by contact angles, did not exhibit any discernible trend.
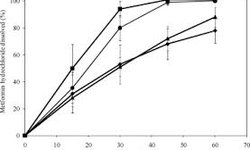
The dissolution behavior of 850 mg metformin hydrochloride tablets in 0.1 N HCl and pH 4.5 acetate buffer, with the addition of 0.01% (w/v) sodium n-octyl sulfate (C8), sodium n-decyl sulfate (C10), or sodium n-tetradecyl sulfate (C14), mirrored that of the control. However, sodium lauryl sulfate (C12) enhanced the dissolution process. At a 0.1% (w/v) concentration, the dissolution enhancement by C12 was mitigated due to a counterbalancing effect between the reduction in surface tension and an increase in hydrophobic ion pairs, characterized by slower diffusivity as observed through nuclear magnetic resonance.
At a 0.1% (w/v) concentration, metformin formed an insoluble salt (1:2 molar ratios) with C10 (pH 1.2), C12, and C14 (pH 1.2 and 4.5), but not with C8. Three competing factors played a role in influencing drug dissolution in the presence of surfactants: the reduction in surface tension of the dissolution media, the formation of ion pairs with low diffusivity, and the creation of an insoluble salt.
Comments
No comments posted yet.
Add a comment