Scientific papers
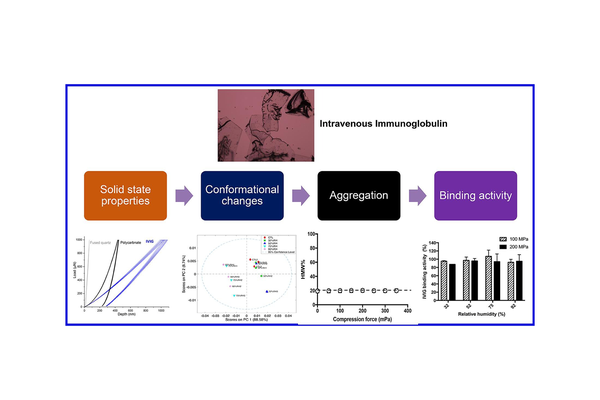
Biological products, encompassing therapeutic proteins, vaccines, and cell-based therapeutics, are witnessing rapid growth in the global market. Monoclonal antibodies, in particular, constitute a substantial portion of the biologics market. When targeting the gastrointestinal tract, the oral delivery route offers numerous advantages, including enhanced patient compliance, convenient administration, and improved stability compared to the parenteral route. To establish the groundwork for the oral delivery of biologics, we conducted a study on the solid-state properties and the impact of compaction pressure, particle size, and storage relative humidity on the stability of immunoglobulin G (IgG).
Utilizing a combination of analytical and biophysical techniques such as size exclusion chromatography and Dynamic Light Scattering for aggregate characterization, Circular Dichroism and solid-state Fourier-transform infrared spectroscopy for evaluating protein secondary structure, and nano-DSC for probing thermal stability, we examined the key factors influencing IgG stability. Our findings indicated that storage relative humidity could induce conformational changes and aggregation in IgG. However, the binding activity of IVIG (intravenous immunoglobulin) did not undergo significant changes with varying relative humidity. While commonly used compaction pressures did not promote protein aggregation, they did noticeably reduce binding activity.
Comments
No comments posted yet.
Add a comment