Scientific papers
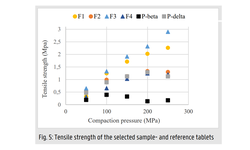
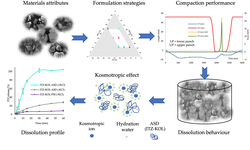
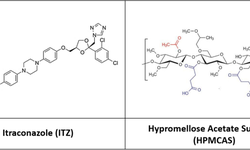
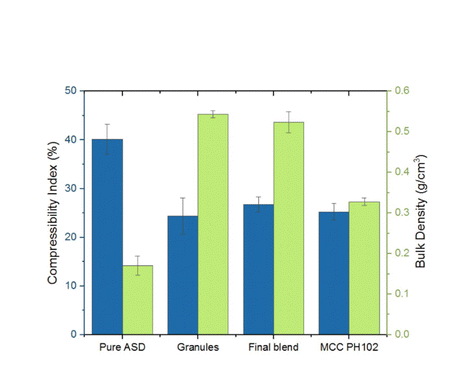
Determining the maximum drug loading in amorphous solid dispersions (ASD) while ensuring sufficient physical stability and release performance is essential for developing ASD-enabled tablets for poorly soluble drugs. Recent research has emphasized the role of the polymer overlap concentration (c*) in maintaining the physical stability of ASD formulations. This study demonstrates the practical application of the c* concept in guiding the development of high drug-loaded ASD tablets, using posaconazole (POS) as a model drug. By leveraging various material-sparing formulation techniques, a breakthrough 50% POS-loaded tablet was created with adequate manufacturability and satisfactory dissolution performance, requiring only 1.5 g of POS and 14 days to complete. The ASD and tablet maintained physical stability for at least six months under ambient conditions and one month at 40 °C.
Comments
No comments posted yet.
Add a comment