Scientific papers
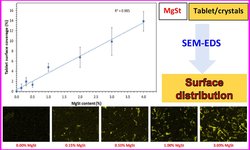
Throughout the development of an 850 mg metformin hydrochloride tablet formulation using wet granulation, the tablets consistently displayed elevated friability levels (>3% w/w) regardless of the origin of extra-granular magnesium stearate (MgSt). The high friability values suggested that the anti-bonding influence of MgSt was too pronounced to be counteracted by the 3.3% w/w povidone binder in the formulation, even with 1.5% w/w residual granule moisture. Attempts to enhance friability by increasing povidone concentration up to 7% w/w yielded limited improvement, with variable friability observed depending on the MgSt source. Examination of MgSt revealed disparities in crystallinity, surface area, and particle morphology among different suppliers. Additionally, a novel bulk yield strength test, assessing the fragmentation tendency of MgSt, proved to be indicative of its performance in the tablet formulation.
In order to enhance the bonding properties of granules, the residual granule moisture was raised to 2% w/w at varying povidone concentrations. Remarkably, at a 2% w/w residual granule moisture content, irrespective of MgSt source, the tablets exhibited a significant reduction in friability (approximately 0.6% w/w), even at the lowest povidone concentration (3.3% w/w). The increased bonding power associated with higher residual granule moisture had a more substantial impact than an elevated povidone concentration in overcoming the anti-bonding effects of magnesium stearate.

Comments
No comments posted yet.
Add a comment