Scientific papers
Objective: Investigate the impact of dissolution medium pH on the immediate release of 850 mg metformin hydrochloride tablets.
Methods: Metformin hydrochloride tablets were manufactured using a conventional wet granulation method, with or without a disintegrant. Tablet dissolution was performed using USP apparatus I at 100 rpm.
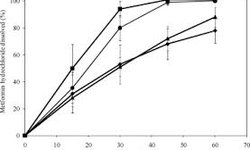
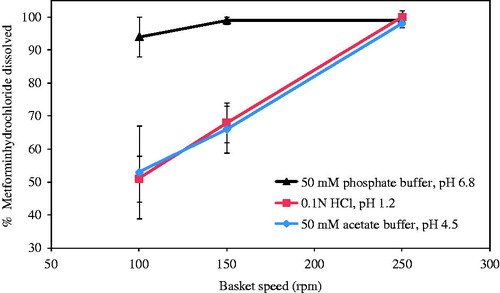
Results: Despite its pH-independent high solubility, metformin hydrochloride tablets exhibited significantly slower dissolution in 0.1 N HCl (pH 1.2) and 50 mM pH 4.5 acetate buffer compared to 50 mM pH 6.8 phosphate buffer, the dissolution medium specified by the USP. This trend was also observed when metformin hydrochloride API was compressed into a round 1200 mg disk. Increasing the basket rotation speed from 100 to 250 rpm resulted in similar dissolution rates for metformin hydrochloride tablets in all three media. The inclusion of 2% w/w crospovidone in the tablet formulation improved dissolution, although the pH-dependent trend persisted. However, the incorporation of 2% w/w croscarmellose sodium led to rapid pH-independent tablet dissolution.
Conclusion: In the absence of a disintegrant in the tablet formulation, dissolution was governed by the erosion-diffusion process. Even for a highly soluble drug, the addition of a super-disintegrant was essential to overcome the diffusion layer limitation and shift the dissolution mechanism from erosion-diffusion to disintegration.
Comments
No comments posted yet.
Add a comment