Scientific papers
This study highlights the potential utility of a novel co-processed excipient, comprising alginic acid and microcrystalline cellulose (Cop AA-MCC), in the formulation of immediate drug release tablets through direct compression. A comprehensive evaluation of the physical, mechanical properties, and disintegration behavior of Cop AA-MCC was conducted, comparing it to commercially available co-processed excipients (Cellactose®, Ludipress®, Prosolv® SMCC HD90, and Prosolv® ODT), as well as to the physical mixture of native excipients (MCC and AA).
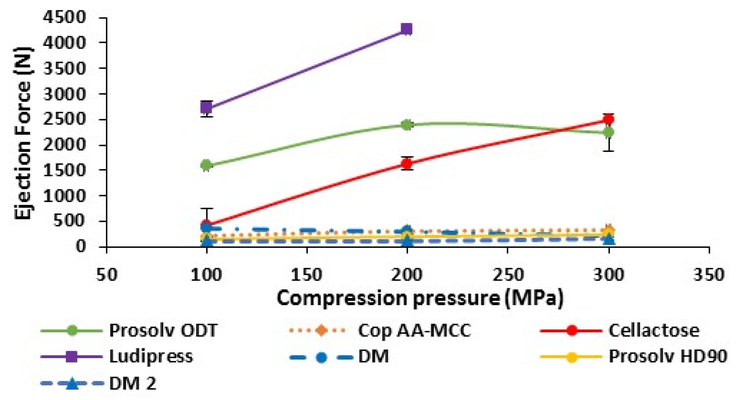
The results demonstrate the favorable performance of Cop AA-MCC in terms of powder flowability, tablet tensile strength, compressibility, and disintegration time. Although this new co-processed excipient exhibited a slightly elevated sensitivity to lubricants, attributed to its more plastic deformation behavior compared to fragmentary, it necessitated a low lubricant quantity due to the notably low ejection force observed during compression. Compression speed and dwell time did not appear to significantly impact the tabletability of Cop AA-MCC.
The study thoroughly compares the performance of Cop AA-MCC with Prosolv® ODT, particularly in terms of tabletability and the dissolution rate of Melatonin. Cop AA-MCC displayed comparable hardness, lower dilution potential, higher lubricant sensitivity, lower ejection force, and a faster release time for Melatonin compared to Prosolv® ODT. In summary, Cop AA-MCC exhibited compelling physical, mechanical, and biopharmaceutical properties, positioning it as a competitive option among commercially available co-processed excipients. Additionally, its straightforward composition and scalable production make this multifunctional excipient highly recommended for direct compression.
Comments
No comments posted yet.
Add a comment