Scientific papers
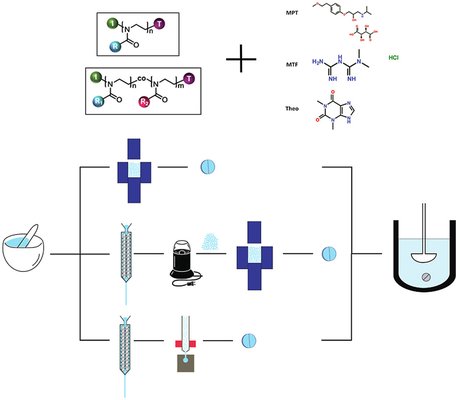
Achieving sustained release of highly dosed active pharmaceutical ingredients (APIs) from a matrix tablet poses a significant challenge. To tackle this issue, this study assessed the efficacy of thermoplastic poly(2-alkyl-2-oxazoline)s (PAOx) as a matrix excipient for producing sustained-release tablets through three processing routes: (a) hot-melt extrusion (HME) combined with injection molding (IM), (b) HME combined with milling and compression, and (c) direct compression (DC). Various PAOx (co-)polymers and polymer mixtures were processed alongside different active pharmaceutical ingredients with varying aqueous solubilities and melting temperatures, including metoprolol tartrate (MPT), metformin hydrochloride (MTF), and theophylline anhydrous (THA). Different grades of PAOx were synthesized and purified, and the impact of PAOx grade and processing technique on in vitro release kinetics was examined.
The use of hydrophobic poly(2-n-propyl-2-oxazoline) (PnPrOx) as a matrix excipient successfully sustained the release of different APIs, even at a 70% (w/w) drug load. While complete THA release within 24 hours was not achieved from the PnPrOx matrix across all processing techniques, the addition of 7.5% w/w of the hydrophilic poly(2-ethyl-2-oxazoline) significantly enhanced THA release. This underscored the importance of blending different PAOx grades. Furthermore, it was demonstrated that the release of THA was consistent from both co-polymer and polymer mixtures with identical polymer ratios.
For MTF, which exhibited fast release from a PnPrOx matrix, the more hydrophobic poly(2-sec-butyl-2-oxazoline) (PsecBuOx) was employed to slow down MTF release. A mixture of the hydrophilic PEtOx and the hydrophobic PsecBuOx allowed precise tuning of MTF formulation release. The study also showcased the ability of PAOx to finely adjust in vivo release. IM tablets containing 70% MTF and 30% PsecBuOx exhibited lower in vivo bioavailability compared to IM tablets with a lower PEtOx concentration (7.5%, w/w) combined with PsecBuOx (22.5%, w/w). Crucially, the in vivo MTF blood levels from sustained release tablets aligned well with the in vitro release profiles. Overall, this research demonstrates that PAOx polymers provide a versatile formulation platform for adjusting the release rate of various APIs, enabling sustained release from tablets with up to 70% w/w drug loading.
Comments
No comments posted yet.
Add a comment















