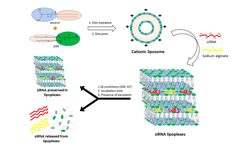
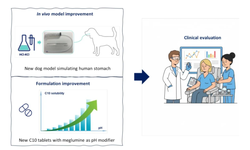
Scientific papers
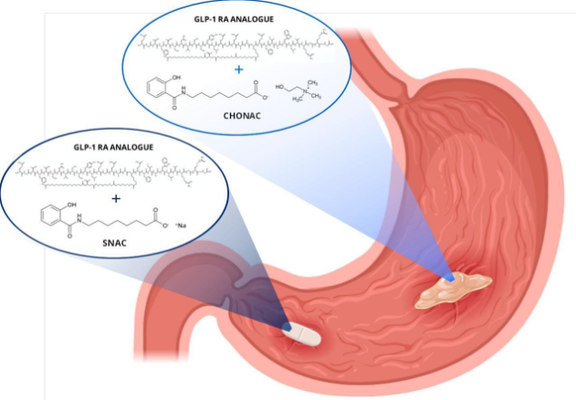
Peptide delivery through oral formulations requires high concentrations of permeation enhancers (PE) to facilitate absorption and often involves fasting between dosing and food intake. Developing formulations that enable rapid absorption would improve convenience but necessitate a quicker onset of action. To address this, we created an ionic liquid (IL) formulation based on salcaprozate—specifically choline salcaprozate (CHONAC)—designed for the gastric absorption of a glucagon-like peptide-1 (GLP-1) analogue.
In vitro studies demonstrated that CHONAC accommodates higher PE concentrations in the same dosage volume, achieving faster release of both the active pharmaceutical ingredient (API) and PE compared to a tablet reference. The formulation showed storage stability for up to three weeks at 4 °C. In vivo studies in rats and anesthetized dogs confirmed quicker peptide absorption with CHONAC compared to reference formulations. However, in awake dogs, although CHONAC enabled earlier API absorption, its overall exposure was lower than the tablet reference. This was primarily due to gastric physiology, which diluted the formulation in the presence of additional fluids and caused rapid liquid transit into the duodenum. In this region, peptides prone to proteolytic degradation, such as the one studied, exhibited negligible absorption, potentially due to CHONAC's reduced permeation-enhancing capability in the duodenum.
Further in vivo research in anesthetized dogs using repeated doses of a liquid salcaprozate-based formulation in the stomach demonstrated the potential for sustained peptide absorption at a consistent rate. In conclusion, enhancing oral peptide bioavailability through gastric delivery requires leveraging the IL formulation's ability to incorporate high PE concentrations and achieve rapid action while ensuring prolonged interaction between the peptide and PE at effective concentrations within the stomach epithelium.
Comments
No comments posted yet.
Add a comment