Scientific papers
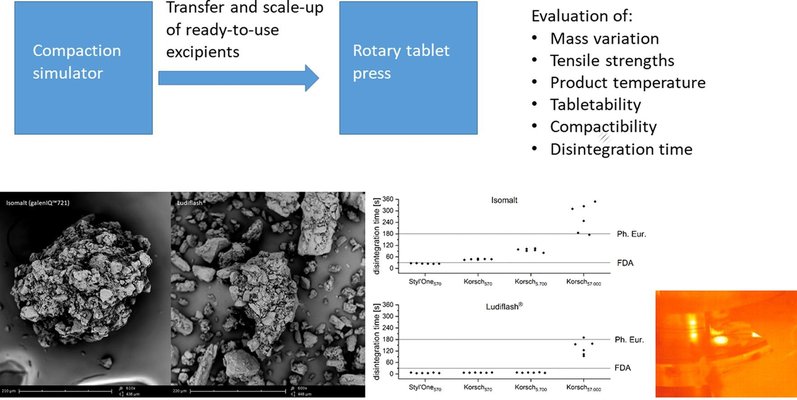
Orodispersible mini-tablets (ODMTs) are emerging as a promising pediatric dosage form, garnering increased attention from the pharmaceutical industry. However, the process of scaling up ODMTs from a compaction simulator to a rotary tablet press, in accordance with FDA and EMA guidelines, has not been previously undertaken and explored. Isomalt (galenIQ™721) and Ludiflash®, both excipients proven suitable for ODMT development, were investigated for transfer and scale-up from a compaction simulator to a rotary tablet press.
ODMTs using isomalt and Ludiflash® were manufactured on the rotary tablet press, with continuous monitoring of product temperature and assessment of residual powder properties in the feed shoe. Key quality attributes such as tensile strength, mass, and disintegration time were thoroughly evaluated. The transfer from compaction simulator to rotary tablet press proved successful, as both excipients exhibited similar disintegration times, tabletability, and compactibility profiles. However, during scale-up, disintegration time showed a significant increase over time for both excipients. Product temperature monitoring revealed that, with an increasing batch size, the product temperature also rises, significantly impacting disintegration time. Remarkably, the properties of ODMTs produced with the residual powder were comparable in tabletability and disintegration time to those produced from fresh powder.
Comments
No comments posted yet.
Add a comment