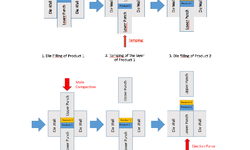
Ease of formulating bilayer tablets and mimicking roller compaction
During the event, MEDELPHARM conducted live demonstrations that displayed the capabilities of the STYL'One Evo. The focus of these experiments included the production of bilayer tablets, combining one layer for immediate release and another for controlled release. This innovative approach offers significant advantages, allowing formulators to merge two active pharmaceutical ingredients (APIs) that may not be compatible with one another or to release APIs at different times, enhancing therapeutic effectiveness.
Compaction simulators like the STYL’One Evo offer several benefits and advantages in the formulation and production of bilayer tablets because they help understand how powders behave, optimize formulations and speed up development. Specifically, the interaction of the two layers during the compaction process can be easily studied, allowing formulators to optimize the mechanical properties of each layer to achieve the desired hardness and disintegration characteristics. By testing different material blends and ratios, compaction simulators help identify the best combinations of active ingredients and excipients for both layers, enhancing the overall performance of the bilayer tablet. The ability to simulate compaction enables the prediction of how each layer will affect the release of active ingredients. This is essential for developing tablets with targeted release profiles, such as immediate or controlled release.
Attendees, including experienced operators of hydraulic simulators, were impressed by the user-friendly design and operational simplicity of the STYL'One Evo. One customer noted how straightforward it was to set up and align punches for oblong tablets, demonstrating the simulator's accessibility for both seasoned professionals and newcomers.
Additionally, the demonstrations included the RocoPack™ software, illustrating how easily the STYL'One Evo can help assess the feasibility of dry granulation, mimic the roll compaction process and determine optimal process parameters for dry granulation with only a few grams of material.
Engagement with acadamia
A notable highlight of MEDELPHARM's presence at AAPS was the engagement with academia. Professors from prestigious universities, including Dr. Tzening Hiew from the University of Iowa, Prof. Calvin Changquan Sun from the University of Minnesota, and Prof. Hoag from the University of Maryland, visited the booth. These universities are equipped with STYL'One Compaction Simulators, integrating these R&D instruments into their research and teaching programs. These partnerships help bridge the gap between research and theoretical knowledge and practical applications in industry, preparing students for careers in the pharmaceutical industry of academia, and providing them with hands-on experience of the tools they may use later in their work.
Poster session
A poster co-written by KORSCH and BASF was also presented during AAPS: “Optimizing tablet formulation through integrated use of digital formulation tools and compaction simulation”
Purpose:
The aim of this study was to optimize the formulation and production process of pharmaceutical tablets using an integrated approach involving algorithm-based formulation tool (ZoomLab®) and a compaction simulator (STYL’One Evo).
This comprehensive workflow was designed to enhance tablet quality and manufacturability by leveraging cutting-edge digital and experimental tools and techniques.
Results:
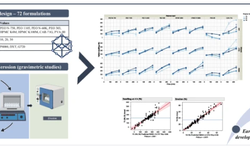
This study uses Kollitab® DC 87L (a co-processed excipient with lactose, Kollicoat® IR, Kollidon® CL-F, and sodium stearyl fumarate) for a blend with excellent flowability, compressibility, and lubrication. The digital formulation tool identified the drug developability classification system (DCS) and predicted powder blend processability for direct compression (DC). It projected processability scores of 7.3 at 20% and 7.0 at 30% drug loading (DL), with values between 5-10 being good for DC.
Experimental runs were based on these predictions. At 114 MPa, the tensile strength was around 1.7 MPa for both DLs, matching experimental results. The compaction simulator revealed declining tablet strength at higher DLs, aligning with the predictions. Discrepancies arose at higher pressures due to increased Ibu concentration.
Ultimately, the digital tool and simulator provided valuable insights for formulation optimization.
Access the whole poster HERE: https://tabletingtechnology.com/papers/optimizing-tablet-formulation-through-integrated-use-of-digital-formulation-tools-and-compaction-simulation/
Conclusion
MEDELPHARM's participation at AAPS PharmSci 360 2024 underscores its dedication to advancing pharmaceutical formulation technologies. Through live demonstrations of the STYL'One Evo and collaboration with esteemed academic institutions, MEDELPHARM continues to play a pivotal role in shaping the future of drug development and tablet formulation.