Scientific papers
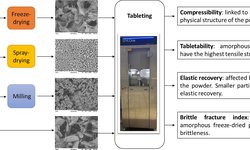
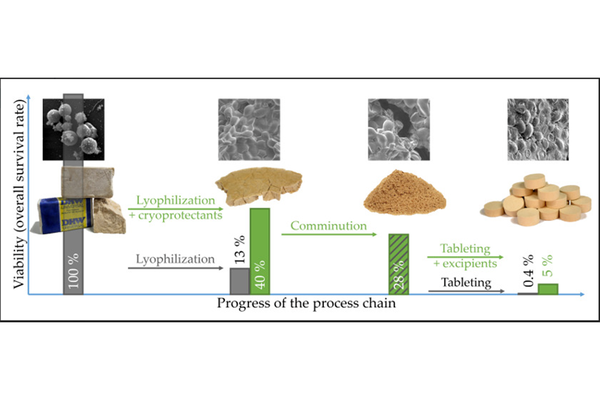
At present, probiotics are predominantly utilized in liquid or semi-solid functionalized foods, resulting in a rapid decline in cell viability. Considering the escalating issue of antibiotic resistance, probiotics hold promise in pharmaceutical development due to their antimicrobial effects. This shift in application introduces new formulation requirements, particularly the need for an extended shelf life achieved through drying, primarily by lyophilization. The production of tablets containing microorganisms via an oral administration process chain is of significant interest, and thus, was the focal point of this study. Lyophilization, as an initial step, exhibited a low cell survival rate of only 12.8%. However, the incorporation of cryoprotectants enhanced survival rates up to 42.9%. Subsequently, the dried cells underwent a gentle milling process. The resulting powder was directly tableted or mixed with excipients such as microcrystalline cellulose, dicalcium phosphate, or lactose. Survival rates during the tableting process ranged from 1.4% to 24.1%, depending on the formulation and applied compaction stress. A more detailed analysis of tablet properties revealed the advantages of excipients in terms of both cell survival and tablet mechanical strength. The maximum overall survival rate throughout the entire manufacturing process exceeded 5%, allowing for doses of 6 × 10^8 colony forming units per gram (CFU/g_total), inclusive of cryoprotectants and excipients.
Comments
No comments posted yet.
Add a comment