Scientific papers
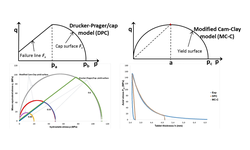
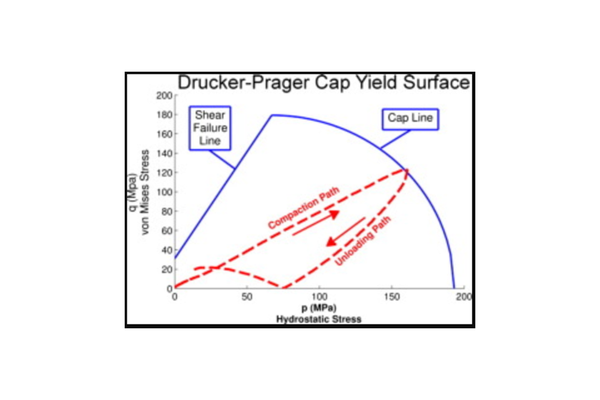
Tablet compaction stands as the predominant method for manufacturing solid dosage forms within the pharmaceutical industry. Despite its widespread application, many commonly employed approaches for assessing the compaction behavior of pharmaceutical materials are empirical and lack a foundation in fundamental material properties. This absence makes it challenging to anticipate potential complications in the compaction process. This study introduces the utilization of a physical model of the compaction process to ascertain pertinent fundamental material properties, enhancing our capability to predict and regulate compaction issues. The parameters of the Drucker–Prager Cap (DPC) model for 14 materials, encompassing excipients, active compounds, and formulations, are outlined. The relationship of the DPC parameters with relative density is determined for each material. Material properties are compared at a uniform relative density of 0.85. Certain formulations are recognized for their compaction risks, such as low tablet strength, high compaction force, or tablet defects. The investigation reveals that examining these model parameters, alongside the stress distribution required for tablet compaction, provides insights into the compaction behavior of these materials. The development of this method facilitates the assessment of compaction risk using a minimal amount of material, reducing the necessity for larger-scale studies.
Comments
No comments posted yet.
Add a comment