Scientific papers
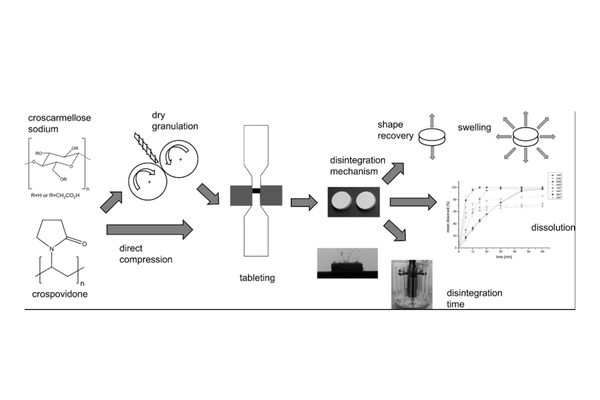
The objective of this study was to assess the functionality of two disintegrants, namely crospovidone and croscarmellose sodium, in tablet formulations processed through roll compaction followed by tableting. The investigation involved studying the impact of different fillers and the influence of sodium lauryl sulfate on the disintegration process using a full factorial design. To enable a direct comparison of disintegrant functionality, center point formulations were also produced through direct compression. Various tablet characteristics, including tensile strength, solid fraction, disintegration time and mechanism, and dissolution profile, were analyzed. The findings lead to the conclusion that the functionality of the disintegrants is compromised by dry granulation. Tablets manufactured through dry granulation and direct compression exhibited differences in both disintegration mechanism and disintegration time. This impact was more noticeable on the functionality of crospovidone compared to croscarmellose sodium. Moreover, sodium lauryl sulfate demonstrated a significant influence on all tablet properties due to its lubricating effect. The choice of filler also had a noteworthy effect on tablet characteristics. These results establish a connection between excipient functionality and drug product properties based on the manufacturing process applied, potentially contributing to an extension of the Manufacturing Classification System to incorporate excipient characteristics.
Comments
No comments posted yet.
Add a comment