Scientific papers
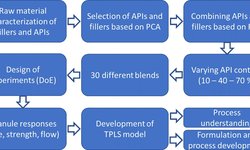
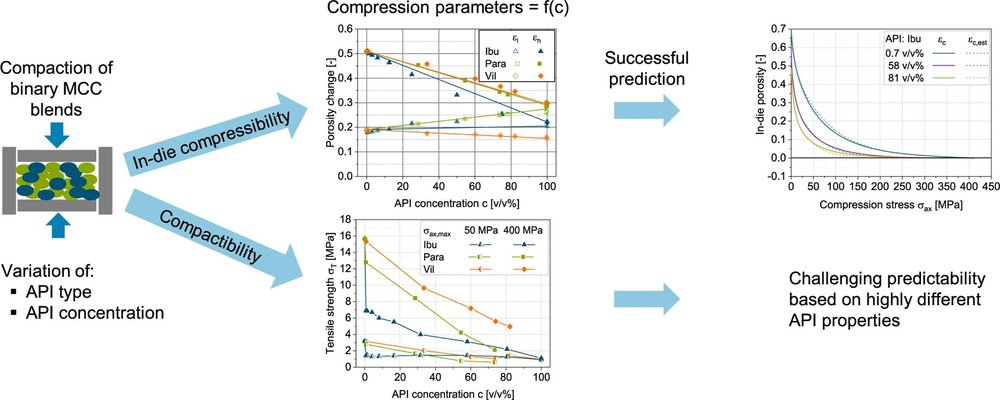
Numerous studies have sought to understand the predictability of compressibility and compactibility in tablet formulations by examining the behavior of individual materials. However, the complexity of the powder compaction process has limited such investigations to a select few materials. To mitigate this complexity, one approach is to extend the scope of research from pure materials to binary powder mixtures. This study specifically focuses on predicting the compressibility and compactibility of binary mixtures comprising an active pharmaceutical ingredient (API) and the excipient microcrystalline cellulose, utilizing three APIs with distinct deformation behaviors.
Through systematic variations in API concentration and type, the study reveals that the in-die compressibility of binary mixtures can be accurately predicted based on the characteristic compression parameters of the raw materials. This prediction is achieved using the extended in-die compression function in conjunction with a volume-based linear mixing rule. Furthermore, considering that tablet porosity (out-of-die) also adheres to a linear mixing rule, the predictability is extended using the methodology proposed by Katz et al. However, it is observed that the influence of API concentration on compactibility or tablet tensile strength is nonlinear and heavily dependent on the deformation behavior of the API, posing challenges to predictability. Notably, neither the approach of Reynolds et al. nor that of Kuentz and Leuenberger can effectively predict compactibility when deviations from a linear mixing rule are evident.
Comments
No comments posted yet.
Add a comment