Scientific papers
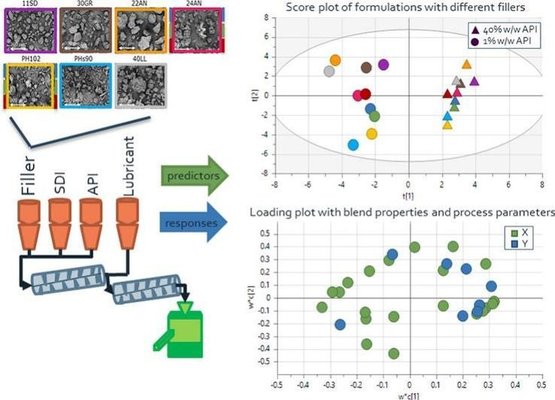
This paper demonstrates the influence of excipient properties on both process parameters and the ultimate tablet properties in a fully integrated continuous direct compression line. The blend properties of low-dose (1% w/w) and high-dose (40% w/w) paracetamol formulations were assessed, and their connection to blending and tableting performance was established through multivariate models, specifically Partial Least Squares analysis (PLS). The feeding behavior was separately analyzed, recognizing that the active pharmaceutical ingredient (API) amount reaching the tablets was influenced by random fluctuations in API feeding behavior.
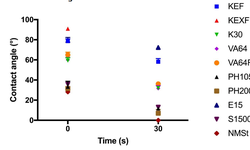
The developed PLS models revealed that formulation behavior was primarily driven by the concentration of the active pharmaceutical ingredient (API), influenced by distinct API properties. Additionally, excipient properties exerted a significant impact on formulation behavior. In general, formulations incorporating microcrystalline cellulose as a filler exhibited enhanced compactability, lower hold-up mass, decreased flowability, and increased cohesion compared to formulations using different lactose grades. The relative performance of formulations with various fillers varied between 1% w/w and 40% w/w drug loading. Granular and spray-dried lactose grades demonstrated improved compactability rankings compared to anhydrous lactose, particularly at higher drug loading, attributed to differences in morphology. The study emphasizes that, in addition to comprehending the impact of excipients on formulation performance, understanding the processability of ingredients is crucial for effective formulation design.
Comments
No comments posted yet.
Add a comment